apparently according to wikipedia, it does not
=.gif)
it is just a bunch of carbon molecules connected all together in double bonds (persnnaly I have never heard of this molecule before

)
they don't mention stuff about graphite, but I have seen photos, ggraphite in this pic
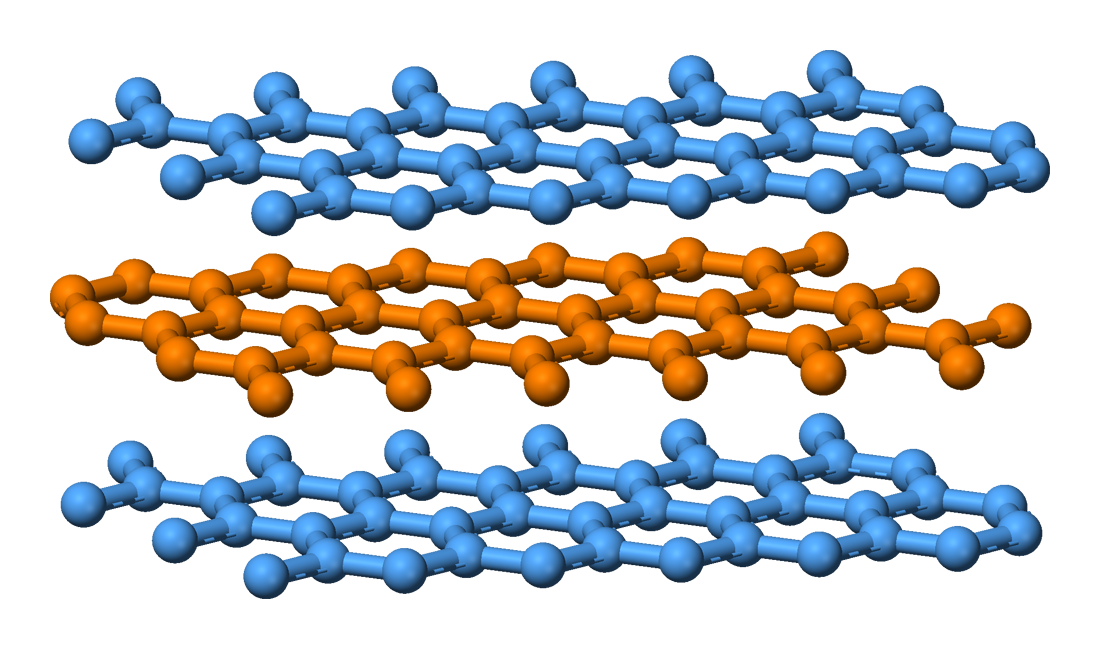
has endings were hydrogen are bonded
graphene on the other hand

does not have any single bonds apparent that bond its carbon molecules to hydrogen
edit: in graphene it seems each carbon makes 3 bonds, that's one double bond and 2 singular to other carbons